Biophysics of bleeding and thrombosis
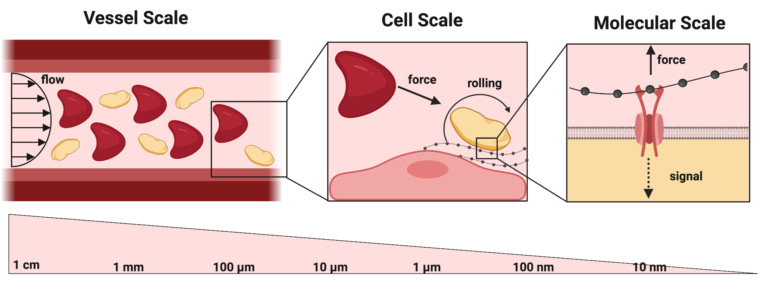
In this line of research we investigate how forces and flows affect blood clot formation in the context of bleeding and thrombosis across the vessel, cellular, and molecular length scales. At the vessel scale we have conducted research on how hematocrit regulates the rate of clot formation in models of arterial and venous thrombosis. At the cellular scale we have used our microfluidic models to study mechanisms of platelet function under a variety of hemodynamic conditions and developed novel methods for studying wall fluxes of platelet agonists and inhibitors. At the molecular scale we are interested in how blood flow regulates the initiation and propagation of coagulation, fibrin polymerization, and von Willebrand factor cleavage. We have made significant contributions in how blood flow regulates the initiation and inhibition of the extrinsic pathway of coagulation. Current work focuses on how specific types of bleeds are influenced by their biophysical environment, which is supported by clinical observations that different disorders result in bleeding in different organs. For example, individuals with hemophilia bleed in their joints and muscles, while individuals with von Willebrand disease bleeding in mucocutaneous tissues. A recurring theme in this work is the use of microsystems coupled with computational fluid dynamics to measure and model mechanical stresses and molecular transport.
This work has been down with a number of collaborators over the years including Jorge Di Paola (Washington University), Alisa Wolberg (UNC-Chapel Hill), Chris Ng (CU-Denver) , Scott Diamond (UPenn), and Aaron Fogelson (Utah) and with the support of the University of Colorado Hemophilia and Thrombosis Center and funded by the American Heart Association, National Science Foundation, and National Institutes of Health.
Related Publications
J. Lin, M.G. Sorrells, W.A. Lam, K.B. Neeves, Physical forces regulating hemostasis and thrombosis: vessels, cells, and molecules in illustrated review. Research and Practice in Thrombosis and Haemostasis, 5 (2021):e12548. DOI: 10.1002/rth2.12548.
M. Bortot, A. Sharif, K. Ashworth, F. Walker, A. Cox, K. Ruegg, N. Clendenen, K.B. Neeves, D. Bark Jr., J. Di Paola. Pathologic shear and elongation rates do not cause cleavage of von Willebrand factor by ADAMTS13 in a purified system. Cellular and Molecular Bioengineering, 13 (2020): 379-390. DOI: 10.1007/s12195-020-00631-2.
M. Bortot, K. Ashworth, A. Sharifi, F. Walker, N. Crawford, K.B. Neeves, D. Bark, J. Di Paola. Turbulent flow promotes cleavage of von Willebrand factor by ADAMTS13. Arteriosclerosis, Thrombosis, and Vascular Biology, 39 (2019): 1831-1842. DOI: 10.1161/ATVBAHA.119.312814.
M. Lehmann, K. Ashworth, M. Manco-Johnson, J.A. Di Paola, K.B. Neeves, C.J. Ng. Evaluation of a microfluidic flow assay to screen for von Willebrand disease and low von Willebrand factor levels. Journal of Thrombosis and Haemostasis, 16 (2018): 104-115. DOI: 10.1111/jth.13881.
B.L. Walton, M. Lehmann, T. Skorczewski, L.A. Holle, J.D. Beckman, J.A. Cribb, M.J. Mooberry, A.R. Wufsus, B.C. Cooley, J.W. Homeister, R. Pawlinski, M.R. Falvo, A.L. Falvo, N.S. Key, A.L. Fogelson, K.B. Neeves, A.S. Wolberg. Elevated hematocrit enhances platelet accumulation following vascular injury. Blood, 129 (2017): 2537-2546. DOI: 10.1182/blood-2016-10-746479.
K. Rana, K.B. Neeves. Blood flow and mass transfer regulation of coagulation. Blood Reviews, 30 (2016): 357-368. DOI: 10.1016/j.blre.2016.04.004.
A.L. Fogelson, K.B. Neeves. Fluid mechanics of blood clot formation. Annual Review of Fluid Mechanics, 47 (2015): 377-403. DOI: 10.1146/annurev-fluid-010814-014513
A.A. Onasoga-Jarvis, T.J. Puls, S.K. O’Brien, L Kuang, H.J. Liang, K.B. Neeves. Thrombin generation and fibrin formation under flow on biomimetic tissue factor rich surfaces. Journal of Thrombosis and Haemostasis,12 (2014): 372-382.
J.L. Sylman, S.M. Lantvit, M.C. VeDepo, M.M. Reynolds, K.B. Neeves. Transport limitations of nitric oxide inhibition of platelet aggregation under flow. Annals of Biomedical Engineering, 41 (2013): 2193-2205.
A.R. Wufsus, N.E. Macera, K.B. Neeves. The hydraulic permeability of blood clots as a function of fibrin and platelet density. Biophysical Journal, 104 (2013): 1812-1823.
K. B. Neeves, D.A.R. Illing, S.L. Diamond.Thrombin flux and wall shear rate regulate fibrin fiber deposition state during polymerization under flow. Biophysical Journal, 98 (2010), 1344-1352.
U.M. Okorie, W.S. Denney, M.S. Chatterjee, K.B. Neeves, and S.L. Diamond. Determination of surface tissue factor thresholds that trigger coagulation at venous and arterial shear rates: amplification of 100 fM circulating tissue factor requires flow. Blood, 111 (2008), 3507-3513.